This guide is intended to familiarise new customers with our
test reports but may also be useful for existing customers to get
more information out of our reports. It covers some of the
frequently asked questions. However, we encourage customers to
contact us directly, either by phone or email, to discuss specific
issues surrounding taking samples or arising from reported
results.
Remember that data included in the report should be treated as
sensitive personal information and must be handled and stored
appropriately.
Our result report can be split into three sections with some
customer specific information at the top followed by the results
and, finally, some notes (if appropriate) towards the bottom.
Clearly, the most important part of the report is the results,
which are presented in a table. But before we discuss them, there
are a few things to point out at the top of the report.
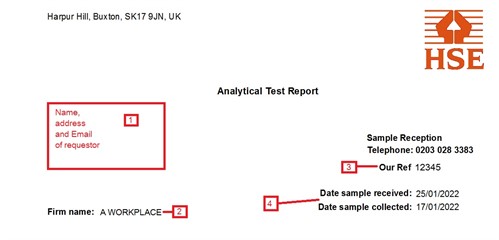
- This will be the person to whom results are sent, not
necessarily the address of the workplace.
- Name of the workplace being sampled.
- Our reference number. This is specific to a particular report -
quoting it will make enquiries about your results easier to
answer.
- Dates that samples were collected by the customer and when they
were received in the lab. Greater than expected difference between
the two may indicate that samples have been delayed in the post.
This may have an impact for some analyses.
Results for each individual are reported on a separate page.
They are presented in table form and, if multiple tests have been
requested, the results are reported in individual columns. In
addition to the test results, this part of the report contains
useful information to help with the interpretation of results.
In order to meet the requirements of data protection (GDPR), we
do not store individual names. Our database uniquely identifies
individuals based on the company, their date of birth and a worker
reference that is supplied by the customer. It can be helpful to
use a consistent worker reference over time to enable easier
comparison with historic data. We encourage customers to use the
simplest possible system to make the process straightforward and
consistent. For example, the worker reference could be the
individual's initials. In rare instances where two or more
individuals share the same initials and year of birth, an
additional identifier, such as a number, could be used. Some
customers prefer to use an alternative unique worker reference,
such as employee number. Ultimately, we are happy to use whatever
identifier works best for each individual client, so long as it
complies with GDPR. It is the duty of the requestor to ensure
compliance with data protection regulations.
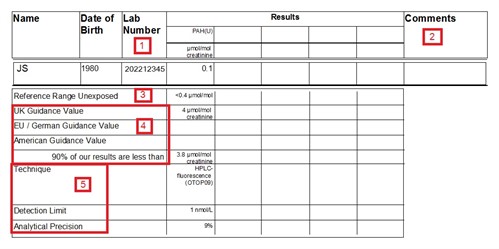
- Our lab number - specific to an individual sample, assigned
when sample is received by the laboratory.
- Comments. e.g. high or low creatinine [1]; other specific
comments as appropriate.
- Reference range - The level of analyte that would be expected
to be found in a non-occupationally exposed population. Many
chemicals are present in the environment, so we are all exposed to
low-levels on a regular basis. Consequently some tests would be
expected to give a background level in all individuals. The
reference range is given in the same units as the reported results
and, unless stated otherwise, it is normally the upper range (95th
percentile) of a substance which might be expected to arise from
environmental (non-occupational) exposure. For some tests,
smokers will have a higher reference range than non-smokers.
If a range applies only to smokers (S) or non-smokers (NS),
it will be signified. Data is cited from "Guidance
on Laboratory Techniques in Occupational Medicine".
- Guidance values - Given in the same units as the results, these
are intended to help the interpretation of results by giving some
context. There are a number of ways of developing a guidance value
and there may not be a UK value for all of the substances for which
we offer biological monitoring. Where available, the corresponding
EU, German or US guidance value is also given. Generally, results
below the guidance value would indicate that exposure is being
adequately controlled. However, for carcinogens and sensitisers,
the aim should be to reduce exposure as low as reasonably
practicable (ALARP) [2]. Where no guidance values are available, we
try to provide contextual data in the form of a 90th percentile of
our lab results (calculated from all of the samples tested over a
recent time period, typically up to 5 years) - this is not official
guidance or necessarily a safe level but what is achievable by
companies using HSE for analysis.
- Information about the analytical technique is provided. This
may be useful when comparing historical data or if results have
been obtained from different laboratories. Bear in mind that
the test detection limit is often given in different units to the
results. Where results are below the Detection Limit, they will be
reported as ND (None Detected).
[1] Creatinine is a small molecule that is excreted as a natural
by-product of metabolism. We measure it in the lab to determine how
concentrated a urine sample is; so that we can correct test results
for concentration. There are established 'normal' levels, but low
creatinine levels can arise from drinking large volumes of fluid,
while high creatinine can often occur as a result of sweating
and/or low fluid intake. Levels outside the normal range are
flagged on the report because creatinine correction may be less
reliable at extremes of urine dilution/ concentration. Rarely, low
creatinine may also indicate sample adulteration.
[2] In the absence of any guidance values, it may still be
possible to set up in-house 'action-levels' for biological
monitoring results. Please contact the lab for advice.
Finally, notes may be added to the report if appropriate, in
addition to some more detailed information about the tests that
have been carried out.

- Relevant comments from the request form such as whether sample
was taken pre-shift or post-shift.
- Where appropriate, comments may be added here by our
scientists, for example if some results exceed the guidance level.
If results exceed the guidance value, checking of control measures
and training then retesting is usually recommended.
- Information about the staff who were involved in lab work and
authorisation of the results. If you wish to discuss the
results, you should contact the Authorised Signatory, quoting the
reference number (Our Ref).